2021 Volume 46 Issue 4 Pages 177-185
2021 Volume 46 Issue 4 Pages 177-185
Chemical modification of the thiol group on protein tyrosine phosphatase (PTP) 1B triggers an activation of epidermal growth factor receptor (EGFR) signaling that is mimicked by environmental electrophiles through S-modification of PTP1B. While activation of PTP1B/EGFR by a single exposure to an electrophile has been established, the effects of combined exposure to electrophiles are unknown. Here, we examined the activation of EGFR signaling by combined exposure to ambient electrophiles in human epithelial carcinoma A431 cells. Simultaneous exposure to 1,2- and 1,4-naphthoquinone (NQ) augmented the S-modification of endogenous and recombinant human PTP1B (hPTP1B). Combined exposure of hPTP1B or A431 cells to 1,2- and 1,4-NQ escalated the inactivation of PTP compared with individual exposure. Phosphorylation of EGFR and its downstream kinase extracellular signal-regulated kinase (ERK) 1/2 by 1,2-NQ exposure was facilitated by simultaneous exposure to 1,2-NQ with 10 µM 1,4-NQ. An EGFR inhibitor diminished the phosphorylation of ERK1/2, indicating that ERK was phosphorylated following EGFR activation by the NQ cocktail. The combined exposure to NQs also accelerated cell death in A431 cells compared with each NQ alone. While no EGFR/ERK activation was seen following 1,4-benzoquinone (BQ) treatment, exposure to 1,4-NQ in the presence of 1,4-BQ increased 1,4-NQ-mediated activation of EGFR. This suggests that the enhancement of 1,4-NQ-dependent EGFR activation by 1,4-BQ is caused by a different mechanism than 1,2-NQ with 1,4-NQ. These results suggest that combined exposure to ambient electrophiles, even at low concentrations, can induce stronger activation of redox signaling than individual exposure. Our findings indicate that combining different electrophiles may produce unexpected effects.
There are various electrophiles in our surrounding environment such as naphthoquinones (NQs) in the vapor phase fraction of ambient and cigarette smoke, benzoquinones (BQ) in diesel exhaust and cigarette smoke, methylmercury in fish, cadmium in rice, aldehydes in vegetables and cigarette smoke, among others, (Eiguren-Fernandez et al., 2008; Jakober et al., 2007; Schmeltz et al., 1977; Kumagai and Abiko, 2017). Electrophilic quinones are produced during photooxidation or biotransformation mediated by drug metabolizing enzymes (Kautzman et al., 2010; Kumagai et al., 2012). For example, naphthalene is biotransformed to its epoxide by cytochrome P450. The epoxide is then hydrolyzed to 1,2-dihydroxy dihydronaphthalene, followed by dehydrogenation by aldo-keto reductase isozymes to form 1,2-dihydroxy naphthalene. This finally undergoes autoxidation to yield 1,2-NQ (Kumagai et al., 2012). One of the major components of cigarette smoke is 1,4-benzosemiquinone, which readily oxidizes to 1,4-BQ (Banerjee et al., 2008; Dey et al., 2010). Thus, we are exposed to such pre-electrophiles in daily life. There is little doubt that multiple exposures to such chemicals can cause a combined effect that worsens their toxicity. However, little research has been performed to elucidate the effects of multiple exposures to electrophiles on cellular responses.
Electrophiles, which can covalently bind to nucleophilic substances in vivo and in vitro, easily modify reactive thiol groups on sensor proteins with a lower pKa value to change the proteins’ functions, leading to activation of its effector molecules (Rudolph and Freeman, 2009; Marnett et al., 2003; Jones, 2008). Protein tyrosine phosphatase (PTP) 1B/epidermal growth factor receptor (EGFR) signaling, a well-known redox signaling pathway, is activated through S-modification of PTP1B by hydrogen peroxide and electrophiles (Lee et al., 1998; Tiganis, 2002; Bae et al., 1997; Tonks, 2003). Our previous study found that 1,2-NQ contracts tracheal smooth muscle in guinea pigs from activation of the phospholipase A2/lipoxygenase/vanilloid receptor signaling pathway, likely through activation of EGFR (Kikuno et al., 2006). In human epithelial carcinoma A431 cells, we found that 1,2-NQ inactivates PTP1B by modification of Cys121, leading to EGFR activation (Iwamoto et al., 2007). Klaus et al. (2010) reported that 1,4-NQ phosphorylates EGFR in HaCaT cells, but the mechanism was still unclear. Our recent study demonstrated that multiple exposures of HepG2 cells to electrophiles, such as 1,2-NQ, 1,4-NQ, and (E)-2-hexenal, facilitates Kelch-like ECH-associated protein 1 (Keap1)/nuclear factor E2-related factor 2 (Nrf2) activation compared with individual exposures. This activation is through S-modification of Keap1 by these electrophiles (Abiko et al., 2021). These observations led us to hypothesize that combined exposure to atmospheric quinones will also cause a joint effect and enhance activation of PTP1B/EGFR signaling. In this study, we aimed to clarify the combined effect of 1,2-NQ and 1,4-NQ as a model of atmospheric quinones on EGFR activation and cytotoxicity in A431 cells. We also examined the effect of monocyclic ambient quinone 1,4-BQ on 1,4-NQ-mediated EGFR activation. The present study will be one of the models of ambient electrophile exposome, belonging to the specific external exposome, which is a concept to refer to the total environmental exposures during whole life (Wild, 2005, 2012).
Epidermal growth factor (EGF), p-nitrophenyl phosphate (pNPP), and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (St. Louis, MO, USA). 1,2-NQ (95% purity determined by HPLC), 1,4-NQ (100% purity determined by GC), and 1,4-BQ (99.1% purity determined by iodometric titration) were purchased from Tokyo Chemical Industry (Tokyo, Japan). PD153035 and anti-PTP1B antibody (# PH01) were acquired from Calbiochem (San Diego, CA, USA). Biotin-(PEAC)5-maleimide (BPM) and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) were obtained from Dojindo Laboratories (Kumamoto, Japan). The anti-EGFR antibody (#2232), anti-phospho EGFR Tyr1068 antibody (#2234), anti-p44/42 MAP kinase antibody [anti-extracellular signal-regulated kinase 1/2 (ERK1/2), #9102], anti-phospho ERK1/2 antibody (#9101), horseradish peroxidase (HRP)-linked anti-rabbit IgG (#7074), and anti-mouse IgG (#7076) secondary antibody were procured from Cell Signaling Technology (Beverly, MA, USA). Fetal bovine serum, GlutaMAX-I, tris(2-carboxyethyl) phosphine hydrochloride (TCEP) and Ni-IDA ProBond were from Corning (Woodland, CA, USA), Gibco (Grand Island, NY, USA), Hampton Research (Aliso Viejo, CA, USA) and Invitrogen (Carlsbad, CA, USA), respectively. The anti-1,4-NQ antibody was prepared as previously reported (Hirose et al., 2012). All other reagents used were of the highest purity available.
Cell cultureThe human epithelial carcinoma A431 cell line (RIKEN Cell Bank, Ibaraki, Japan) was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Wako, Osaka, Japan) supplemented with 10% fetal bovine serum, antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin, Wako), and 2 mM GlutaMAX-I in a 5% CO2 humidified atmosphere at 37°C. For each experiment, A431 cells were grown in a 35-mm dish or 96-well plate until reaching 80-100% confluence. Before treatment with electrophiles, the cells were starved in serum-free DMEM for 24 hr.
Purification of recombinant human PTP1B proteinRecombinant human PTP1B (hPTP1B) was prepared as previously described (Iwamoto et al., 2007). Briefly, E. coli BL21 cells with human 37-kDa PTP1B (NH2-terminal 321 residues) in a pET15b vector were grown to an absorbance of 0.6 at 600 nm in LB broth containing 100 µg/mL ampicillin at 37°C, and then was incubated with 200 µM isopropyl-1-thio-β-D-galactopyranoside at 15°C for 21 hr. The E. coli, which were harvested by centrifugation, were sonicated on ice in lysis buffer [50 mM Tris-HCl (pH 7.5)-100 mM NaCl-10 mM 2-mercaptoethanol-5% glycerol] for 30 min and centrifuged (105,398 × g, 1 hr, 4°C). The supernatant was applied to a Ni-IDA ProBond column at 4°C. The column was extensively washed with an equilibrate buffer [50 mM Tris-HCl (pH 7.5)-100 mM NaCl-10 mM 2-mercaptoethanol]. hPTP1B was then eluted with imidazole and each fraction was monitored by checking the absorbance at 280 nm and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The fractions containing hPTP1B were reduced by 10 mM dithiothreitol (1 hr on ice), and the purified hPTP1B was stored at -80°C in 50 mM Tris-HCl (pH 7.5)-10 mM dithiothreitol, in which dithiothreitol was removed before use using PD SpinTrap G-25 (GE Healthcare™, Chicago, IL, USA). The protein concentration was determined using the Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s protocol.
Phosphatase assayPhosphatase assays were performed as previously described (Iwamoto et al., 2007) with slight modifications. Briefly, A431 cells were exposed to electrophiles for 30 min. The cells were washed with ice-cold phosphate-buffered saline (PBS) and collected in RIPA buffer [50 mM Tris-HCl (pH 8.0), 0.1% SDS, 150 mM NaCl, 1% Nonidet P-40, and 0.5% deoxycholate]. The total cell lysate (20 µg of protein) was incubated with 4 mM pNPP in 40 mM Tris-HCl (pH7.5)-80 mM NaCl-0.08% bovine serum albumin for 30 min at 37°C, and then an equal volume of 2 M NaOH was added. The absorbance of the released p-nitrophenol (pNP) was determined at 405 nm. For hPTP1B, the protein [10 μM in 50 mM Tris-HCl (pH 7.5)-0.1 mM EDTA] was incubated with electrophiles at 25°C for 10 min. Then, the reaction mixture (0.5 μg of PTP1B) was incubated with 4 mM pNPP in 0.1 mM EDTA-100 mM acetate buffer (pH 5.5) for 10 min at 25°C, followed by addition of an equal volume of 2 M NaOH. The absorbance was measured at 405 nm.
Western blottingThe cells, which were exposed to various compounds, were washed three times with ice-cold PBS and collected by scraping into lysis buffer [20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, and 1 mM Na3VO4, 1% protease inhibitor (Sigma)]. These samples were sonicated on ice followed by centrifugation (15,000 × g, 10 min, 4°C). Protein concentration of the supernatant was determined using the bicinchoninic acid assay (BCA, Thermo Fisher Scientific, Waltham, MA, USA). The protein concentration of each sample was normalized before mixing with a half volume of SDS-PAGE loading buffer [6% SDS, 62.5 mM Tris-HCl (pH 6.8), 24% glycerol, 15% 2-ME, and 0.015% bromophenol blue] and heated for 5 min at 95°C. The proteins were separated by SDS-PAGE and then electro-transferred onto polyvinylidene difluoride membranes (Pall Corporation, Pensacola, FL, USA) at 2 mA/cm2 for 1 hr. The membranes were blocked with 5% skim milk in TTBS [20 mM Tris-HCl (pH 7.5), 150 mM NaCl, and 0.1% Tween 20] for 1 hr at room temperature, incubated with the indicated primary antibodies overnight at 4°C, and then reacted with HRP-linked secondary antibodies at room temperature for 1 hr. The immunoreactive proteins on the membranes were detected using Chemi-Lumi One L (Nacalai Tesque, Kyoto, Japan). The results are representative of three or more experiments. Band intensity was measured using ImageJ software (Wayne Rasband, National Institutes of Health, USA).
BPM-labeling assayThe electrophilic modification of protein by NQs was detected using a BPM-labeling assay, as described previously (Toyama et al., 2013; Abiko et al., 2015). Briefly, 10 µM of hPTP1B was reacted with electrophiles for 60 min at 25°C, followed by further incubation with 200 µM BPM in 50 mM Tris-HCl (pH 8.0) for 30 min at 37°C. Each sample was heated in half the volume of loading buffer [6% SDS, 62.5 mM Tris-HCl (pH 6.8), 24% glycerol, 50 mM TCEP, and 0.015% bromophenol blue] for 5 min at 95°C, and SDS-PAGE and western blotting was then performed. After treatment with electrophiles, the cells were washed three times with ice-cold PBS and collected by scraping in RIPA buffer [50 mM Tris-HCl (pH 8.0), 0.1% SDS, 0.5% deoxycholate (w/v), 1% NP-40 (w/v), and 150 mM NaCl] containing 100 µM BPM and 1% protease inhibitor cocktail. These samples were incubated on ice for 1 hr and centrifuged at 15,000 × g for 10 min at 4°C to collect supernatant. The protein concentration was then determined by a BCA assay. The sample was incubated with half the volume of the loading buffer for 5 min at 95°C, and then western blotting was performed. After blocking with 5% skim milk in TTBS, the membrane was incubated with an HRP-linked anti-biotin antibody at room temperature for 1 hr. The immunoreactive proteins on the membranes were detected using Chemi-Lumi One. Representative data from three independent experiments are shown. Band intensity was measured using ImageJ software.
Cytotoxicity assayAfter exposure to compounds in 96-well plates, A431 cells were treated with 5 mg/mL MTT solution (1/20, v/v) for 20 min in a CO2 incubator at 37°C. After removal of the medium, DMSO (100 μL/well) was added, followed by incubation at room temperature for 30 min. The absorbance was measured at 540 nm using a spectrophotometer (iMark Microplate Absorbance Reader, Bio-Rad).
Data analysisAll data are presented as the mean ± SE from at least three independent experiments. Statistical significance was assessed using one- or two-way ANOVA, followed by Dunnett multiple-comparison test using GraphPad Prism version 6.0 software (San Diego, CA, USA). P < 0.05 was considered statistically significant.
As shown in Fig. 1A, exposure of A431 cells to 1,4-NQ modified cellular proteins in a concentration-dependent manner detected with a polyclonal antibody against 1,4-NQ (Hirose et al., 2012). A BPM-labeling assay, which can indirectly detect protein S-modification (Toyama et al., 2013; Abiko et al., 2015), revealed that co-exposure of A431 cells to NQs (1,2-NQ and 1,4-NQ) increased S-modification of the cellular proteins compared with exposure to each NQ alone (Fig. 1B). This suggests that these electrophiles could be attacked by thiol groups on proteins leading to gained effects and/or responses against electrophiles compared with individual exposure.

Covalent modification of cellular protein by ambient electrophiles in A431 cells. (A) The cells were exposed to 1,4-NQ for 30 min and total cell proteins were subjected to western blot analysis with indicated antibodies. Each value is the mean ± S.E. of three independent experiments. **, P < 0.01 vs. 0 µM. (B) The cells were exposed to 1,2-NQ, 1,4-NQ, or a mixture of equal concentration of 1,2-NQ with 1,4-NQ for 30 min. The S-modification was detected with a BPM assay. The proteins on the SDS-PAGE gel were stained with coomassie brilliant blue (CBB). Each value is the mean ± S.E. of three independent experiments. *, P < 0.05.
We have reported that 1,2-NQ modifies a sensor protein PTP1B through its reactive thiol, which disrupts its enzymatic activity and thereby activates EGFR (Iwamoto et al., 2007). We then assessed S-modification and inhibition of recombinant hPTP1B by 1,4-NQ that were detected by a BPM assay and pNPP assay, respectively. BPM-bound hPTP1B levels were significantly decreased during reaction with 1,4-NQ in a concentration-dependent manner (Fig. 2A), indicating an increased amount of 1,4-NQ-induced S-modification. Corresponding to this, 1,4-NQ inhibited enzymatic activity of hPTP1B (Fig. 2B). Twenty µM 1,4-NQ, but not 1,2-NQ (20 µM), significantly modified thiol group of hPTP1B (Fig. 2A and 2C). However, 10 µM 1,2-NQ strongly inhibited the enzymatic activity of hPTP1B compare to 10 µM 1,4-NQ (Fig. 2B and 2D). While 2.5 or 5 µM of 1,4-NQ did not significantly inhibit hPTP1B activity, co-incubation of 1,4-NQ with 1,2-NQ (2 or 8 µM) tended to augment this inhibition (Fig. 2E). Such enhancement of inhibition of cellular phosphatase activity was also seen in the A431 cells (Fig. 3).
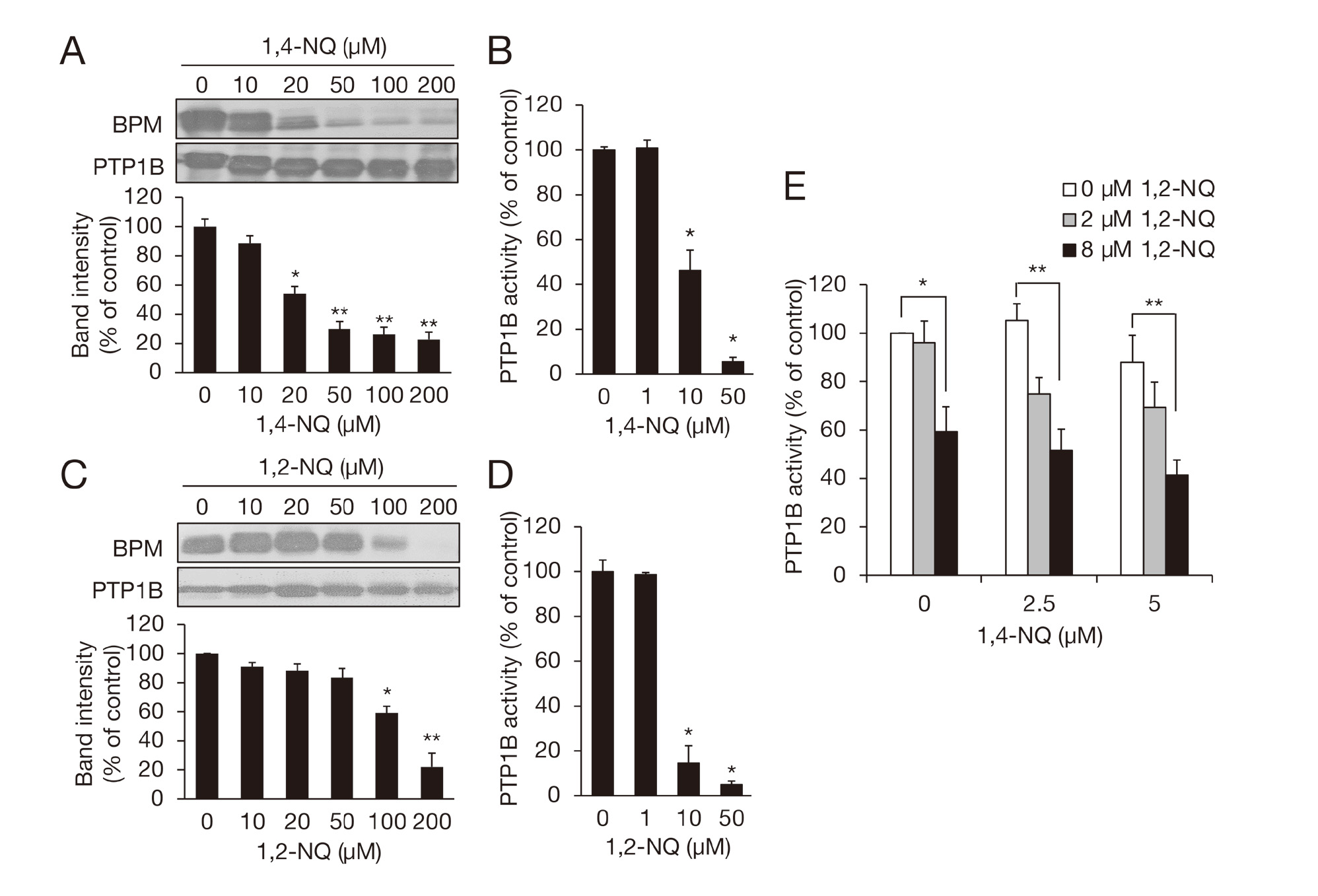
S-Modification of hPTP1B and inhibition of enzymatic activity by single and combined exposure to 1,2-NQ and 1,4-NQ. hPTP1B was exposed to 1,4-NQ (A) or 1,2-NQ (C) for 1 hr at 25°C, and then S-modification of the protein was analyzed with a BPM assay. Each value is the mean ± S.E. of three independent experiments and expressed as a percentage of band intensity of the control. *, P < 0.05; **, P < 0.01 vs. 0 µM. hPTP1B was exposed to 1,4-NQ (B) or 1,2-NQ (D) for 10 min at 25°C, and then PTP1B activity was measured with pNPP assay. Each value is the mean ± S.E. of three independent experiments. *, P < 0.01 vs. 0 µM. (E) hPTP1B was exposed to 1,4-NQ (0-5 µM) and 1,2-NQ (0-8 µM) for 10 min at 25°C, and then PTP1B activity was measured. Each value is the mean ± S.E. of three independent experiments. *, P < 0.05; **, P < 0.01.

Effect of single and combined exposure to 1,2-NQ and/or 1,4-NQ on PTP activity in A431 cells. The cells were exposed to 1,2-NQ (0-50 µM), 1,4-NQ (0-50 µM), or a mixture of 1,2-NQ and 1,4-NQ (0-50 µM each) for 30 min. The PTP activity in the cell lysate was determined with pNPP assay. Each value is the mean ± S.E. of three independent experiments. *, P < 0.05; **, P < 0.01.
Next, we observed that 1,4-NQ could phosphorylate EGFR and ERK1/2 (Fig. 4) and confirmed our previous findings with 1,2-NQ (Supplemental Fig. S1), which clarified activation of PTP1B/EGFR signaling by 1,2-NQ exposure in A431 cells (Kumagai et al., 2012; Iwamoto et al., 2007). These results indicate that combined exposure of the cells to 1,2-NQ and 1,4-NQ can facilitate EGFR activation compared with individual exposure. As expected, 1,2-NQ-mediated phosphorylation of EGFR and ERK1/2 was enhanced by co-treatment with 1,4-NQ (10 µM) (Fig. 4 and Fig. 5A). This effect was diminished by adding an EGFR inhibitor PD153035 (Fig. 5B), indicating that PTP1B/EGFR/ERK1/2 were activated by exposure to the NQ cocktail. In a separate study as a model of an environmental electrophile exposome, it was also shown that multiple exposures to ambient quinones consisting of 1,2-NQ, 1,4-NQ, and phytochemical (E)-2-hexenal can potentiate Nrf2 activation. It can also induce its downstream genes, such as heme oxygenase-1 and glutamate-cysteine ligase modifier subunit, presumably through modification of its negative regulator Keap1 in HepG2 cells (Abiko et al., 2021). An NQ cocktail (1,2-NQ with 1,4-NQ, 5 or 10 µM each) exacerbates cytotoxicity by (E)-2-hexenal in HepG2 cells (Abiko et al., 2021). In response to this, we also tested the contribution of the combined exposure to cell death in A431 cells. Individual exposure to 5 µM of 1,2-NQ or 1,4-NQ increased the cell viability, whereas mixtures of non-toxic concentrations of 1,2-NQ (5 or 15 µM) and 1,4-NQ (5 or 15 µM) significantly induced cell death compared with single exposures (Fig. 6). These observations are agreement with our previous studies that 1,2-NQ and 1,4-NQ modify sensor proteins (PTP1B, PTEN) with reactive thiol groups, leading to inhibition of their activities, thereby substantially activating effector molecules (EGFR, Akt) involved in cell proliferation and cell survival (Iwamoto et al., 2007; Abiko et al., 2017) at lower doses; however, increasing dose of these ambient electrophiles are associated with disruption of such redox signaling pathways and cell damage through extensive modification of the cellular proteins.
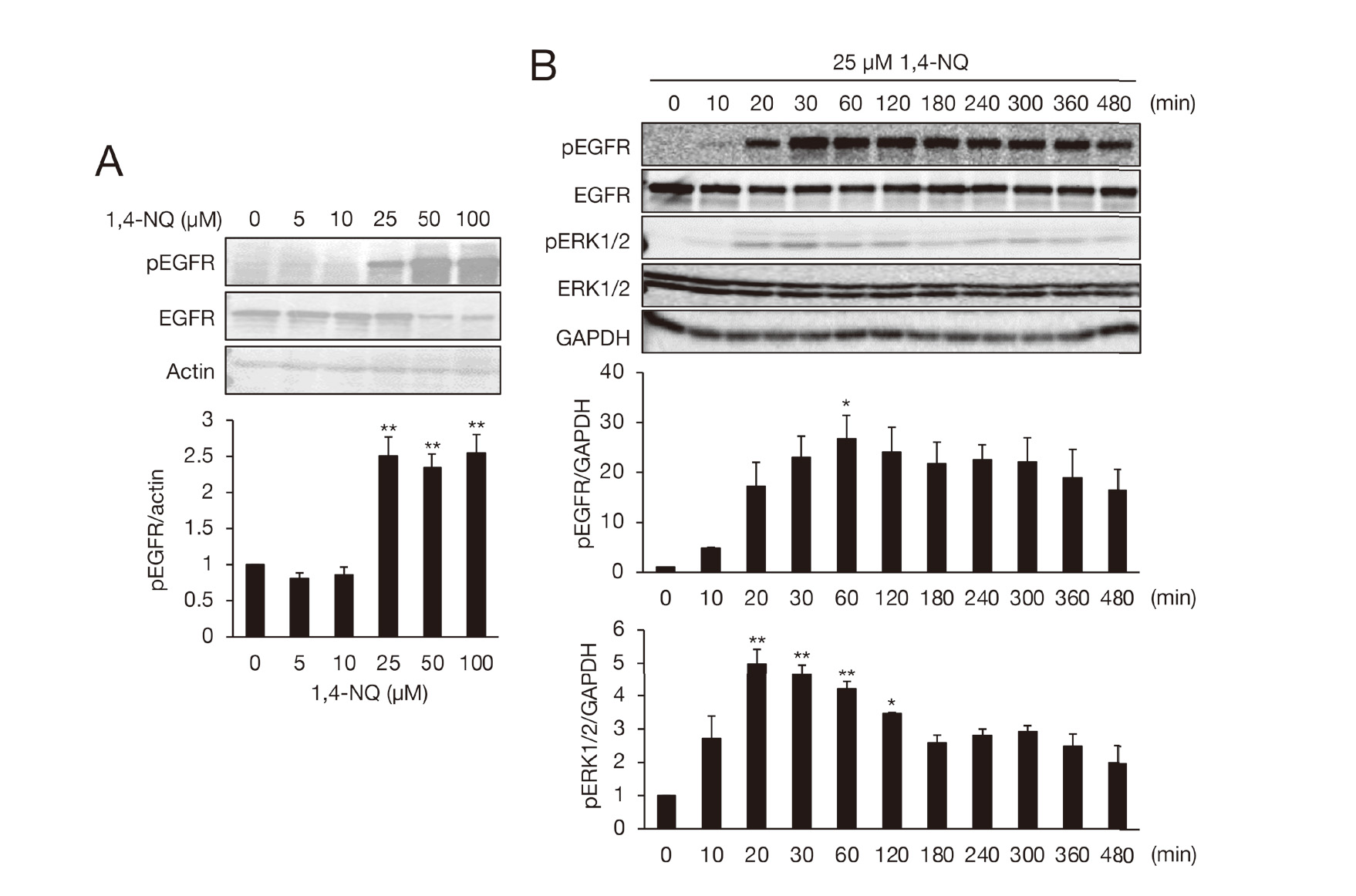
Activation of EGFR and its downstream kinase ERK1/2 by 1,4-NQ in A431 cells. (A) The cells were exposed to 1,4-NQ (0-100 µM) for 30 min. (B) The cells were exposed to 25 µM 1,4-NQ for 0-480 min. The total cell proteins were subjected to western blot analysis with indicated antibodies. Each value is the mean ± S.E. of three independent experiments. *, P < 0.05; **, P < 0.01.

Effect of 1,4-NQ on 1,2-NQ-mediated activation of EGFR/ERK1/2 signaling in A431 cells. (A) The cells were exposed to 1,2-NQ (0-100 µM) with or without 10 µM 1,4-NQ for 30 min. (B) The cells were pretreated with PD153035 (0 or 1 µM) for 2 hr, and then exposed to 1,2-NQ (0-100 µM) with 10 µM 1,4-NQ for 30 min. The total cell proteins were subjected to western blot analysis with indicated antibodies. Each value is the mean ± S.E. of three independent experiments. *, P < 0.05; **, P < 0.01.
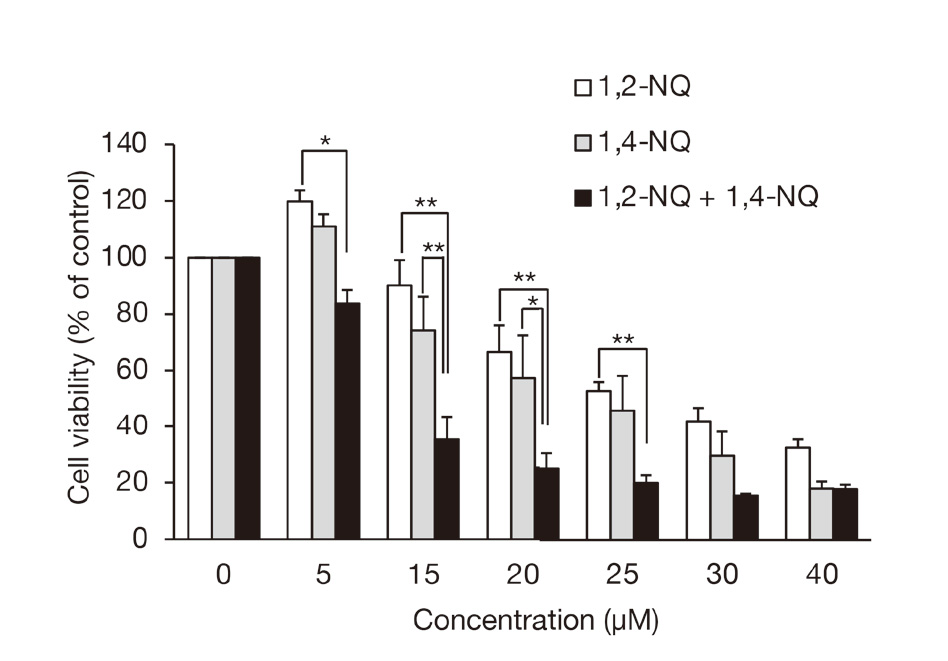
Cell viability during exposure to 1,2-NQ, and 1,4-NQ alone or together. A431 cells were exposed to 1,2-NQ (0-40 µM), 1,4-NQ (0-40 µM), or both (0-40 µM each) for 24 hr, and then MTT assay were performed. Each value is the mean ± S.E. of three independent experiments. *, P < 0.05, **, P < 0.01.
Monocyclic electrophilic quinone 1,4-BQ is also capable of binding to nucleophilic moieties such as thiol groups (Kleiner et al., 1998; Mbiya et al., 2013). 1,4-BQ tended to inhibit the activity of cellular PTPs (Fig. 7A). However, 1,4-BQ (5-100 µM) did not phosphorylate EGFR and ERK1/2 in this condition (Fig. 7B). 1,4-BQ undergoes NADPH-cytochrome reductase-catalyzed redox cycling of its hydroquinone to yield reactive oxygen species (ROS) only at high pH value (Boersma et al., 1994), suggesting that such redox cycling is unlikely to occur at physiological conditions. Yet, it has been reported that 1,4-BQ activated ERK/MAPK signaling through ROS production in human promyelocytic leukemia HL-60 cells (Ruiz-Ramos et al., 2005). Abdelmohsen et al. (2003) have demonstrated that 1,4-BQ can activate EGFR by mechanisms other than inactivation of PTPs. The authors speculated that depletion of cellular glutathione levels by 1,4-BQ impaired substantial reduction of PTPs. Taken together, several unknown factors are related to the activation of signaling by the monocyclic quinone. Interestingly, as shown in Fig. 7B, 1,4-NQ-mediated activation of EGFR was significantly stimulated by 1,4-BQ in a concentration-dependent manner, indicating that the joint effect of 1,4-NQ with 1,4-BQ was caused by different mechanisms than binary exposure of NQs, although 1,4-BQ itself did not affect EGFR activation.

Effect of 1,4-BQ on 1,4-NQ-mediated inhibition of PTP activity and activation of EGFR/ERK1/2 signaling in A431 cells. (A) The cells were exposed to 1,4-BQ (0, 50, 200 µM) with or without 10 µM 1,4-NQ for 30 min, and then PTP activity was measured with pNPP assay. (B) The cells were exposed to 1,4-BQ (0, 5, 25, 100 µM), 1,4-NQ (0, 5, 10, 25 µM), or 1,4-BQ (0, 5, 25, 100 µM) with 10 µM 1,4-NQ for 30 min. The total cell proteins were subjected to western blot analysis with indicated antibodies. Each value is the mean ± S.E. of three independent experiments. *, P < 0.05.
Simultaneous exposure of rat liver epithelial cells to N-acetyl cysteine (NAC, 30 mM) with 1,4-BQ (100 µM) negated 1,4-BQ-mediated EGFR activation (Abdelmohsen et al., 2003), suggesting that such an effect is likely from trapping of 1,4-BQ mediated by NAC. In our preliminary examination, we found that extracellular levels of Cys, as well as its persulfide that is capable of capturing NQs, markedly declined following 1,4-BQ (25 µM) exposure (Abiko Y et al., unpublished observation). Thus, a possible explanation for this unique observation is that 1,4-BQ is trapped by Cys and its persulfide, resulting in formation of sulfur adducts (Nishida et al., 2012). Additionally, the concomitant increase in intracellular levels of 1,4-NQ is associated with enhancement of EGFR activation. Further studies are needed to clarify the specific effects of combined exposure of electrophiles on redox signaling. We show here that combined exposure to electrophiles, even at low concentrations, can induce stronger activation of redox signaling compared with individual electrophile exposure. It is possible that, in some case, combination of different chemicals, even those with electrophilic properties, can bring unexpected effects.
In conclusion, while exposome, which is a new paradigm to encompass the totality of human environmental exposures from conception onwards, complementing the genome, was firstly proposed by Christopher Wild in 2005 (Wild, 2005), he subsequently mentioned that three categories of non-genetic exposures consisting of 1) general external, 2) specific external and 3) internal in exposome may considered (Wild, 2012). Of interest, electrophiles, which are able to covalently modify protein thiols, resulting in alteration in the protein functions have been recognized as “priority components”. Since there are so many electrophiles (e.g., crotonaldehyde, acrolein, methyl vinyl ketone) other than 1,2-NQ, 1,4-NQ and 1,4-BQ in the atmosphere, further investigation based on combined exposure to ambient electrophiles are required to develop specific external exposome study.
This work was supported by a grant-in-aid (#18H05293 to Y.K. and #20K12180 to Y.A.) for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. We thank J. Iacona, Ph.D., from Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript.
Conflict of interestThe authors declare that there is no conflict of interest.